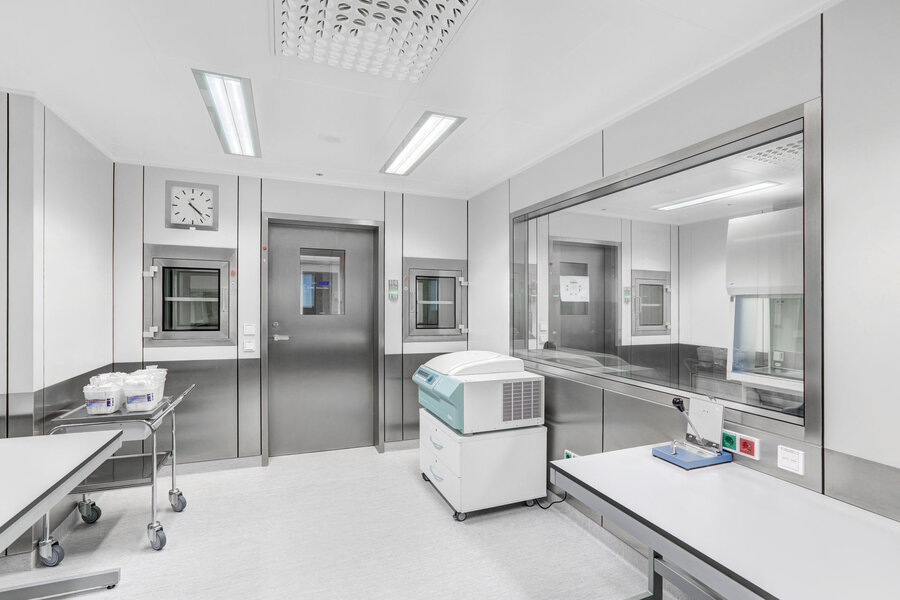
As such, certain room requirements compliant with GMP need to be met, i.e. laboratory rooms must meet classes D to A according to EU GMP or other cleanliness requirements according to ISO 14644. Examples include the implementation of suitable pressure levels and modular room systems with correspondingly hygienic and smooth surfaces. With its expertise in this research area, HT can support you in this process from planning to implementation.




Safety from planning to operation
HT Group offers reliable planning of GMP laboratories for all areas of application and BIM-supported project execution on request.
All technical building systems and parameters are taken into account (TGA / MSR / monitoring), and special requirements can also be easily integrated thanks to the modular, variable system.
The specially developedGMP material airlocks in variable sizes are used for the safe entry and exit of materials, samples or pharmaceuticals. The sluice systems are available as active, partially active or passive material pass-throughs, multi-chamber airlocks or trolley sluice for transferring large and heavy materials and can optionally be equipped with EI60 fire protection.
A requirement with explosion protection in accordance with the ATEX directive for the chemical industry, pharmaceuticals or gas plants, for example, can also be implemented.
Qualification tests complete the construction project:
How are the cleanliness classes verified?
- To define the cleanliness class, the particle concentration of the room air and the air exchange rate are measured during qualification
- ISO 14644-3 and VDI 2083-3 define various measurement methods and limit values (= maximum permissible particle concentration per m³)
Pressure level measurement according to ISO 14644-3 and VDI 2083-3
- Rooms according to pressure level plan
- Personnel airlocks
- Material airlocks